
Copper has been shown to be required by a specific mouse olfactory receptor, MOR244-3, which is highly responsive to MTMT as well as to various other thiols and related compounds. Katz and co-workers showed that MTMT functioned as a semiochemical, activating certain mouse olfactory sensory neurons, attracting female mice. (Methylthio)methanethiol (MeSCH 2SH MTMT) is a strong-smelling volatile thiol, also detectable at parts per billion levels, found in male mouse urine. Human sweat contains ( R)/( S)-3-methyl-3-mercapto-1-ol (MSH), detectable at 2 parts per billion and having a fruity, onion-like odor. These compounds are detectable by the human nose at concentrations of only 10 parts per billion. The spray of skunks consists mainly of low-molecular-weight thiols and derivatives. The odors of thiols, particularly those of low molecular weight, are often strong and repulsive. Many thiols have strong odors resembling that of garlic.

There are several ways to name the alkylthiols: Thiols have a lower dipole moment relative to their corresponding alcohols. In contrast, O−H bonds in hydroxyl groups are more polar. ĭue to the small difference in the electronegativity of sulfur and hydrogen, an S−H bond is moderately polar. For CH 3S−H, the BDE is 366 kJ/mol (87 kcal/mol), while for CH 3O−H, the BDE is 440 kJ/mol (110 kcal/mol). The S−H bond is much weaker than the O−H bond as reflected in their respective bond dissociation energy (BDE). In the solid or liquids, the hydrogen-bonding between individual thiol groups is weak, the main cohesive force being Van der Waals interactions between the highly polarizable divalent sulfur centers.
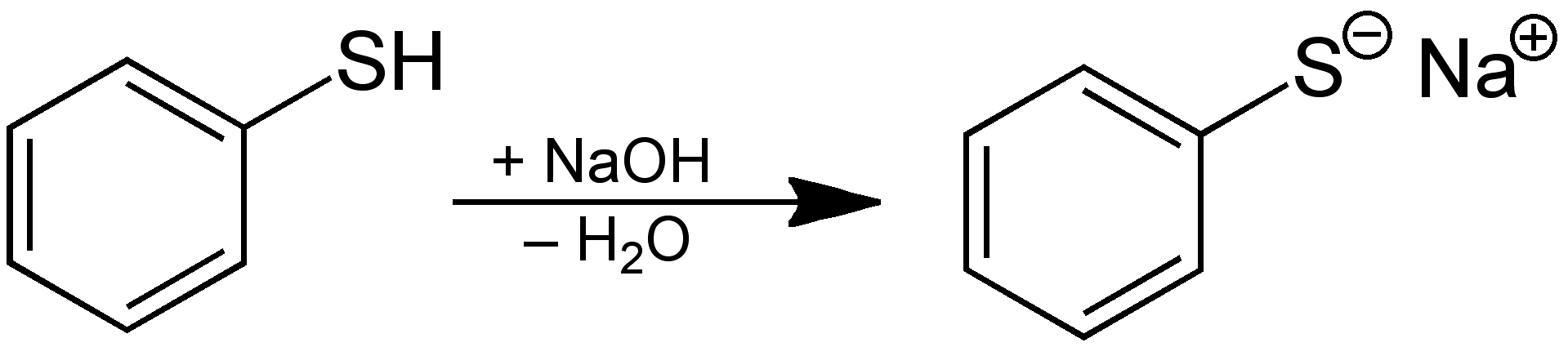
The C−S−H angles approach 90° whereas the angle for the C−O−H group is more obtuse. Because sulfur atoms are larger than oxygen atoms, the C−S bond lengths – typically around 180 picometres in length – are about 40 picometers longer than a typical C−O bond. Thiols and alcohols have similar connectivity. Thiols of the structure R−SH are referred to as Alkanethiols or Alkyl thiols, in which an alkyl group (R) is attached to a sulfhydryl group (SH). Thiols are sometimes referred to as mercaptans ( / m ər ˈ k æ p t æ n/) or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin mercurio captāns ('capturing mercury') because the thiolate group ( RS −) bonds very strongly with mercury compounds. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant.
Many thiols have strong odors resembling that of garlic or rotten eggs.

Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl ( −OH) group of an alcohol), and the word is a blend of " thio-" with "alcohol". The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. In organic chemistry, a thiol ( / ˈ θ aɪ ɒ l/ from Ancient Greek θεῖον (theion) ' sulfur' ), or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. Thiol with a blue highlighted sulfhydryl group.


 0 kommentar(er)
0 kommentar(er)
